
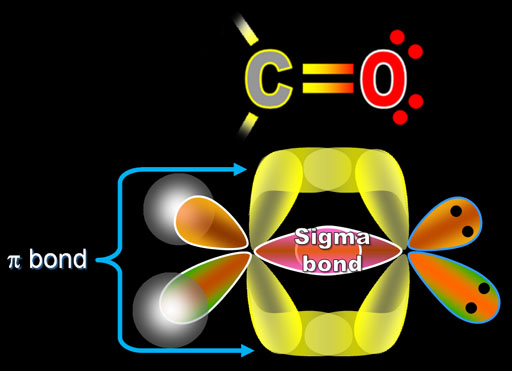
That means, we have already got the lewis structure of XeF 4. In chemistry, sigma bonds ( bonds) are the strongest type of covalent chemical bond. There are no charges on atoms in above sketch and do not need to worry about reducing charges to obtainīest stable structure. Mark charges on atoms and check the stability and minimize charges on atoms by converting lone pairs to bonds< Then all remained lone pairs are finished and there are no more lone pairs to mark. Therefore, then mark those two electrons pairs on xenon atom. Now, only 2 (14-12) lone pairs are remaining.Then all four fluorine atoms will take 12 lone pairs. Each fluorine atom will take three lone pairs. XeF 4, fluorine atoms are the outside atoms. Usually as a theory, those remaining electron pairs should be first marked on outside atoms.There are already 4 sigma bonds in the above drawn basic sketch.Remember that, there are total of 18 electron pairs to mark on atoms as lone pairs and bonds. Mark lone pairs on xenon and fluorine atomsĪfter deciding the center atom and basic sketch of XeF 4, we can start to mark Therefore, xenon becomes the center atom and each fluorine atom is joint with xenon atom. However, from our experience we know that there is a very low possibility that fluorine atomĬannot be a center atom because fluorine's maximum valence is 1 (fluorine cannot make two or more bonds). To be the center atom, ability of having greater valance and being a electropositive element are Selection of center atom and sketch of XeF 4 molecule XeF 4, total pairs of electrons are 18 (=36/2) in their valence shells. If the element (such as N or O) has a double bond directly on it, then its lone pair doesn’t count in the conjugation system if there’s a single bond, then its lone pair is counted.

Total electron pairs are determined by dividing the number total valence electrons by two. Do lone pairs count as pi bonds A delocalized lone pair does participate in the conjugated system and will be counted in the pi electrons. Pairs = σ bonds + π bonds + lone pairs at valence shells

Now we know how many electrons includes in valence shells of xenon and fluorine atom. Is a group IA element and has 8 electrons in its last shell (valence shell).įluorine is a group VIIA element in the periodic table and contains 7 electrons in their last shell. There are only two elements in XeF 4 xenon and fluorine. From the point of view of localized bond pairs and lone pairs, predictions about bond. Check the stability and minimize charges on atoms by converting lone pairs to bonds until most stableįind total number of electrons of the valance shells of XeF 4 The technique of hybridization allows prediction of strong directed bonds.Find total number of electrons of the valance shells of xenon and fluorine atoms.Look the figures to understand each step. If you are a beginner to lewis structure drawing, follow these sections slowly and properly to understand There are several steps to draw the lewis structure of XeF 4. The order of repulsion between electron pairs is as follows: Lone pair-lone pair > lone pair-bond pair > bond pair-bond pair.Steps of drawing lewis structure of XeF 4
\ has two lone pairs.Īccording to VSEPR theory the valence electron pair surrounding an atom tends to repel each other. Let’s solve the given question one by one The VSEPR theory is used to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. Lone pairs are found in the outermost electron shell of atoms. Lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond and is sometimes called an unshared pair or non-bonding pair. The number of lone pairs present in the central atom and the number of electrons involved in bonding determine the shape. Hint: The number of lone pair and bond atoms present in between the atoms determine the shape of the molecule.


 0 kommentar(er)
0 kommentar(er)
